At Drug Plastics, we want our customers to have all of the information needed to make the right choices when designing cost-effective, efficient, and eye-catching product packaging. Selecting the correct bottle type is only the beginning of the story. The type of closure selected plays a large role in the design too – in order for a packaging system to perform as expected, the closure must work properly with the bottle. Selecting an improper closure liner can cause major issues like product damage and loss, waste, and of course, unnecessary costs.
Important Considerations
Container Material Type – The material type of the bottle you choose will determine the liner options. The most common material types used for plastic bottles are HDPE, LDPE, PET, Polyvinyl Chloride (PVC), PS, and “treated” glass. Liners seal differently on these various material types: the bottle material type you choose plays a large part in the closure liner type you select.
Product Being Packaged – The industry the product serves determines the liner type. For example, the food and beverage, pharmaceutical, personal care, and chemical industries require packaging components to meet certain FDA-approved requirements. It is important to know which requirements apply to your product.
Product Characteristics – How is your product delivered: dry (like a solid dose medication or powder); a viscous material? Is it in liquid form or highly acidic? Does it include an aggressive ingredient? Will the seal work with the form and reactivity of the contents? Protecting your product is paramount and providing all the information about your product’s characteristics will help determine which closure liner will perform best.
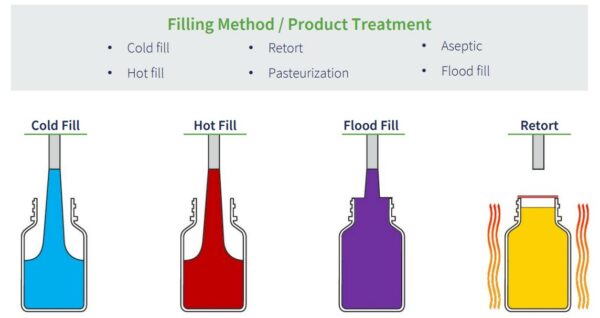
Product Filling Process – The method used to fill packaging with liquid contents also impacts the closure liner choice. In addition, the product treatment can affect the seal type that can be used. Cold fill; hot fill; retort; pasteurization; aseptic; and flood fill are the most common type of filling processes.
Removal Characteristics – There are several scenarios for liner removal: a clean land area on the bottle when the seal is removed; piercing the liner; and a tamper evident liner. Answers to these questions will help you determine which liner meets your requirements.
- Clean Peel – Easy to peel and remove in one piece with no residual material left on the land area of the bottle.
- Tamper Indicating Peel – Easy to peel and remove in one piece, with residual material left on the land area of the bottle.
- Puncturable – Punctures easily, with residual material left on the land area of the bottle.
- Puncture-Resistant – Requires a tool to puncture, with residual material left on the land area of the bottle.
Removal Features – A mechanism that is part of the liner to assist the user when opening. Choose from several forms:
- Side Tabs – Die cut/punched part of the liner itself, available in one or multiple tabs. These tabs also help retain the liner in the closure.
- Top Tabs – No contact with seal or product surface because it is applied to the top of the liner. Provides a larger half-moon shaped tab on top of the liner, offering more surface area for the consumer to remove the liner.

Closure Size – The dimensions of the closure liner is based on the bottle and closure sizes selected for your packaging.
Origin of Closure Liner Materials
Know where liners are manufactured and where the raw materials used to produce them are sourced is important. If liners are manufactured in non-regulated countries, or if the raw materials used to produce the liner are not sourced in a regulated country, you could potentially be non-compliant with regulations established by agencies like the FDA.
When you work with us, you are assured that every closure liner we use is manufactured in, and the raw materials used in their production, are sourced in the USA or Canada. The liners are FDA-approved, just like all of our bottles and closures. You can have confidence that your products will be packaged appropriately and meet the strict regulatory requirements for your industry. At both of our closure manufacturing facilities in Phoenix, AZ and Edinburgh, IN, we cut liners from sheet stock to exact size specifications, and produce closures in a variety of sizes.
Save Costs by Choosing the Best Closure Liner
The liner can be an expensive part of the bottle and closure packaging system. Picking the wrong liner that does not work correctly, or is not made for the specific type of contents could damage the product and result in lost product, waste, or increased liability for your company.
These scenarios add potential costs. In addition, over-specifying the liner means paying more for something that really isn’t needed. For example, opt for a simple foam liner for a product that requires it instead of selecting a liner with unnecessary facings. You will avoid incurring additional costs that reduce your profitability.
We Can Help
At Drug Plastics, we help our customers design the best packaging option for their products. Solutions that are cost-effective, enhance a brand, reduce liability, and improve the bottom line. We can do the same for you. Our 60+ years of experience help create the best product packaging. Ready to discuss your project? Speak with someone immediately at 610-367-5000.
Read the first article in this series: “Understanding Closure Liners and the Materials Used to Produce Them“.
For additional and more in-depth information about closure liners, we invite you to visit https://www.seligsealing.com/.